Strategies for controlling pathogen growth
There are a number of strategies for the control of pathogens in fish and fishery products. They include:
- Managing the amount of time that food is exposed to temperatures that are favorable for pathogen growth and toxin production (covered in this chapter; for Clostridium botulinum, in Chapter 13, and for Staphylococcus aureus in hydrated batter mixes in Chapter 15);
- Killing pathogens by cooking (covered in Chapter 16), pasteurizing (covered in Chapter 17), or retorting (covered by the low acid canned foods regulations, 21 CFR 113);
- Controlling the amount of moisture that is available for pathogen growth, water activity, in the product by drying (covered in Chapter 14);
- Controlling the amount of moisture that is available for pathogen growth, water activity, in the product by formulation (covered in Chapter 13);
- Controlling the amount of salt or preservatives, such as sodium nitrite, in the product (covered in Chapter 13);
- Controlling the level of acidity, pH, in the product (covered by the acidified foods regulations, 21 CFR 114 for shelf-stable products; and for refrigerated acidified products in Chapter 13).
Note: The use of irradiation for fish or fishery products has not been approved by FDA. Irradiated fish and fishery products may not be distributed in the U.S.
Managing time and temperature of exposure
The time/temperature combinations that will ensure safety in your product are dependent upon a number of factors, including:
- The types of pathogens that are expected to be present and able to grow in your product. See information contained in Step #11.
- The infective or toxic dose of these pathogens or their toxins. The infective or toxic dose is the total number of a pathogen, or the total amount of a toxin, that is necessary to produce human illness. The dose often varies considerably for a single pathogen based on the health of the consumer and the virulence (infective capability) of the particular strain of the pathogen.
For many of the pathogens listed in Table #A-1 (Appendix 4) the infective dose is known or suspected to be very low (from one to several hundred organisms). These include: Campylobacter jejuni, Escherichia coli, Salmonella spp., Shigella spp., and Yersinia enterocolitica. The infective dose for other pathogens, such as Vibrio vulnificus, Vibrio parahaemolyticus and Listeria monocytogenes is not known. In the case of both of these categories of pathogens it is advisable to prevent any significant growth. Stated another way, product temperatures should be maintained below the minimum growth temperature for the pathogen or should not be allowed to exceed that temperature for longer than the lag growth phase (i.e the slow growth phase during which pathogens are acclimating to their environment) of the pathogen at those temperatures.
Still other pathogens (e.g. Vibrio cholerae) require large numbers in order to cause disease or require large numbers in order to produce toxin (e.g. Staphylococcus aureus, Clostridium perfringens, Bacillus cereus). The infective dose of Vibrio cholerae is suspected to be 1,000,000 total cells. S. aureus toxin does not normally reach levels that will cause food poisoning until the numbers of the pathogen reach 100,000 to 1,000,000/gram. Clostridium perfringens does not produce toxin in the human gut unless at least 100,000,000 total bacteria are consumed. Limited growth of these pathogens may not compromise the safety of the product. However, time/temperature controls must be adequate to prevent growth before the stage of the infective or toxic dose is reached. For example, the prudent processor will design controls to ensure that the numbers of S. aureus do not exceed 10,000/gram.
- The numbers of these pathogens that are likely to be present. This is highly dependent upon the quality of the harvest water, how the raw material was handled before it was delivered to your plant, and the effectiveness of your sanitation control program. As a practical matter, the initial number of pathogens is of limited importance when you calculate critical limits for pathogens that have a low infective dose. Therefore, you will be designing a critical limit that prevents any significant growth.
On the other hand, for those pathogens that have a relatively high infective dose, the initial number of pathogens may be significant.
STEP #11: Determine if this potential hazard is significant.
At each processing step, determine whether "pathogen growth and toxin production as a result of time/temperature abuse" is a significant hazard. The criteria are:
1. Is it reasonably likely that unsafe levels of pathogens will be introduced at this processing step (do unsafe levels come in with the raw material or will the process introduce them)?
It is reasonable to assume that pathogens of various types, including those listed in Table #A-1 (Appendix 4), will be present on raw fish and fishery products and non-fishery ingredients. They may only be present at low levels or only sporadically, but even such occurrences warrant consideration because of the potential for growth and toxin production.
Pathogens also may be introduced during processing, even after cooking (as described in Step #10). Well designed sanitation programs (prerequisite programs) will minimize the introduction of pathogens. However, in most cases it is not reasonable to assume that they will fully prevent the introduction of pathogens. For this reason, controls should be in place to minimize the risk of pathogen growth after the cook step.
2. Is it reasonably likely that pathogens will grow to unsafe levels and/or produce toxin at this processing step?
In order to answer this question you must first determine which of those pathogens that are reasonably likely to be present in your product would be able to grow if proper time/temperature controls are not maintained. Consider:
- the moisture available to support pathogen growth in the product (water activity);
- the amount of salt and preservatives in the product;
- the acidity (pH) of the product;
- the availability of oxygen (aerobic vs anaerobic) in the product;
- the presence of competing spoilage organisms in the food.
Table #A-1 (Appendix 4) provides guidance on some conditions of a food that limit the growth of those pathogens that are most relevant to fish and fishery products. This table can help you to decide if a particular pathogen will grow in your food if it is temperature abused.
Certain pathogens grow well in temperature abused raw fish (e.g. raw molluscan shellfish) and others do not. Those which grow well in temperature abused raw fish include: Vibrio vulnificus, Vibrio parahaemolyticus, Vibrio cholerae, and Listeria monocytogenes. Those which ordinarily do not grow well, because they compete poorly with the normal spoilage bacteria, include: Campylobacter jejuni, pathogenic strain of Escherichia coli, Salmonella spp., Shigella spp., Staphylococcus aureus, and Yersinia enterocolitica.
Most will grow well in temperature abused cooked fish if their growth is not controlled by means such as drying, salting, or acidification because competing bacteria are destroyed by the cooking process. Others may grow if the natural condition of the raw fish is changed, such as through salting or reduced oxygen packaging.
Remember that you should consider the potential for time/temperature abuse in the absence of controls. You may already have controls in your process that minimize the potential for time/temperature abuse that could result in unsafe levels of pathogens or toxins. This and the following steps will help you determine whether those or other controls should be included in your HACCP plan.
Time/temperature abuse that occurs at successive processing steps (including storage steps) may be sufficient to result in unsafe levels of pathogens or toxins, even when abuse at one step alone would not result in such levels. For this reason, you should consider the cumulative effect of time/temperature abuse during the entire process. Table #A-2 (Appendix 4) provides guidance about the kinds of time/temperature abuse that may cause a product to be unsafe.
In summary, under ordinary circumstances (e.g. without data to the contrary) you should consider that it is reasonably likely that a pathogen in Table #A-1 (Appendix 4) will grow to an unsafe level or produce toxin in your product at a particular processing step if all of the following conditions are met:
- It is reasonably likely to be present (see question 1, above);
- It is not inhibited by a condition of the food (see Table A-1 [Appendix 4] );
- If your product is raw fish (e.g. raw molluscan shellfish): it will grow in temperature abused raw fish (see information in this question, above);
- It is reasonably likely that, in the absence of controls, cumulative time/temperature abuse conditions such as those described in Table #A-2 (Appendix 4) could occur, and the processing step could contribute significantly to that cumulative abuse.
3. Can the growth to unsafe levels and/or toxin production of pathogens, which is reasonably likely to occur, be eliminated or reduced to an acceptable level at this processing step? (Note: If you are not certain of the answer to this question at this time, you may answer "No." However, you may need to change this answer when you assign critical control points in Step #12.)
"Pathogen growth and toxin formation as a result of time/temperature abuse" should also be considered a significant hazard at any processing step where a preventive measure is, or can be, used to eliminate (or reduce the likelihood of occurrence to an acceptable level) the hazard, if it reasonably likely to occur.
Step #10 discusses a number of pathogen control strategies. This section covers control of pathogen growth and toxin production that occurs as a result of time/temperature abuse. Preventive measures for such growth can include:
- Maintaining product under refrigeration and controlling refrigeration temperatures;
- Proper icing;
- Controlling the amount of time that product is exposed to temperatures that would permit pathogen growth and/or toxin production;
- Rapidly cooling fish;
- Making sure that the temperature of incoming microbiologically sensitive (e.g. raw and cooked ready-to-eat fishery products) was properlycontrolled during transportation.
List such preventive measures in Column 5 of the Hazard Analysis Worksheet at the appropriate processing step(s).
If the answer to either question 1, 2 or 3 is "Yes" the potential hazard is significant at that step in the process and you should answer "Yes" in Column 3 of the Hazard Analysis Worksheet. If none of the criteria is met you should answer "No." You should record the reason for your "Yes" or "No" answer in Column 4. You need not complete Steps #12 through 18 for this hazard for those processing steps where you have recorded a "No."
It is important to note that identifying this hazard as significant at a processing step does not mean that it must be controlled at that processing step. The next step will help you determine where in the process the critical control point is located.
Intended use
In determining whether a hazard is significant you should also consider the intended use of the product, which you developed in Step #4. FDA is not aware of any HACCP controls that may exist internationally for the control of pathogens in fish and fishery products that are intended to be fully cooked by the consumer or end user before consumption, other than a rigorous sanitation regime as part of either a prerequisite program or as part of HACCP itself. The Seafood HACCP Regulation requires such a regime. The proper application of sanitation controls is essential because of the likelihood that any pathogens that may be present in seafood products are introduced through poor handling practices (e.g. by the aquacultural producer, the fisherman, or the processor).
FDA is interested in information regarding any HACCP controls beyond sanitation that may be both necessary and practical for the control of pathogens in fish and fishery products that are intended to be fully cooked by the consumer or end user before consumption. However, the agency makes no recommendations in this Guide and has no specific expectations with regard to such controls in processors' HACCP plans. The agency plans to develop Good Manufacturing Practice guidelines for harvest vessels and for aquaculture, in an effort to minimize the likelihood that these operations will contribute pathogens to fish and fishery products.
If your product is intended to be fully cooked by the consumer or end user before consumption, you should enter "No" in Column 3 of the Hazard Analysis Worksheet for each of the processing steps. For each "No" entry briefly explain in Column 4 that the hazard will be controlled by the consumer or end user cook. In this case, you need not complete Steps #12 through 18 for this hazard.
One exception to this general rule relates to the formation of heat-stable toxins, such as that which is produced by Staphylococcus aureus. The toxin produced by S. aureus is not destroyed by cooking, even retorting. Its formation should, therefore, be prevented in all fish and fishery products. However, as previously mentioned, S. aureus does not grow well in raw fish, unless the growth of competing spoilage organisms is inhibited (e.g., by salting or vacuum packaging). Bacillus cereus also produces a heat-stable toxin.
STEP # 12: Identify the critical control points (CCP).
For each processing step where "pathogen growth and toxin formation as a result of time/temperature abuse" is identified in Column 3 of the Hazard Analysis Worksheet as a significant hazard, determine whether it is necessary to exercise control at that step in order to control the hazard. Figure #A-2 (Appendix 3) is a CCP decision tree that can be used to aid you in your determination.
The following guidance will also assist you in determining whether a processing step is a CCP for this hazard:
Is there a cook step, a pasteurization step, or a retorting step later in your manufacturing process?
1. If there is, you may in most cases identify the cook step, pasteurization step, or retorting step as the CCP. Processing steps prior to cooking, pasteurization, or retorting will then not usually need to be identified as CCPs for this hazard.
Example:
A cooked shrimp processor could set the critical control point for "pathogen growth and toxin formation as a result of time/temperature abuse" at the cook step, and would not need to identify each of the processing steps prior to cooking as critical control points.
Guidance for this pathogen control strategy (e.g. heat treatment) is contained in: Chapter 16 (cooking); Chapter 17 (pasteurization); and, the Low Acid Canned Foods Regulations, 21 CFR 113 (retorting).
There are two important limitations to this strategy. One is that the cooking, pasteurizing, or retorting process must be sufficient to eliminate the pathogens of concern. If it is not, time/temperature control may still be necessary at the processing steps at which growth may occur.
The other limitation is that certain toxins (e.g. Staphylococcus aureus and Bacillus cereus toxins) are heat stable. Heat treatment, including retorting, may not be adequate to eliminate the toxin once it is formed. In this case time/temperature control may be necessary at the processing steps at which growth and toxin production may occur.
2. If there is no cook step, pasteurization step, or retorting step later in the process, then it may be necessary to identify each processing step at which you have identified this hazard as significant as a critical control point for the hazard. Exposure of the product to temperatures that will permit growth and/or toxin formation should be controlled at these steps.
Example:
A crab meat processor identifies a series of post-cook processing and storage steps (e.g. backing, picking, packing, and refrigerated storage) as presenting a reasonable likelihood of pathogen growth and toxin formation. The processor does not subject the product to a final pasteurization process and acknowledges that it may be consumed without further cooking. The processor controls temperature during refrigerated storage, and time of exposure to unrefrigerated conditions during the processing steps. The processor identifies each of the post-cook processing and storage steps as CCPs for this hazard.
In this case, you should enter "Yes" in Column 6 of the Hazard Analysis Worksheet for each of those processing steps. This control approach is referred to as "Control Strategy Example 1" in Steps 14-18.
Note: Rather than identify each step as an individual CCP when the controls are the same at those steps, it may be more convenient to combine into one CCP those steps that together contribute to cumulative time/temperature exposure.
It is important to note that you may select a control strategy that is different from that which is suggested above, provided that it assures an equivalent degree of safety of the product.
Following is guidance on processing steps that are likely to be identified as critical control points for this hazard because time/temperature control is necessary to control pathogen growth and/or toxin production. The guidance is divided into two finished product types, because the hazard control strategies differ. The two finished product types are cooked, ready-to-eat and raw, ready-to-eat.
Cooked, ready-to-eat
These products are cooked by the processor and may be eaten with no further cooking by the consumer. Examples include: cooked crab meat, lobster meat and crayfish meat, surimi-based analog products, seafood salads, and hot-smoked fish. Note that smoked fish is also covered in Chapter 13.
Cooked, ready-to-eat products, especially fabricated products, may develop pathogen hazards as a result of cross contamination and growth. Contributing factors to this risk are manual handling steps, multiple ingredients, room temperature processing, and multiple cooling steps. Cumulative exposure to temperature abuse after the cook step must be taken into consideration.
A final pasteurization step (e.g. pasteurized crabmeat) or retorting step (e.g. canned, hot-smoked salmon) may make identification of critical control points at prior processing steps unnecessary for most pathogens. However, neither pasteurization nor retorting is sufficient to inactivate Staphylococcus aureus toxin. Bacillus cereus also produces a heat-stable toxin. For this hazard you should consider the possibility that the toxin will be produced before the final heat treatment, and control toxin formation, if necessary.
In some cases cooked, ready-to-eat ingredients, such as lobster meat, pasteurized crabmeat, smoked fish, and surimi-based analog products, are received for storage, or assembly into a product that will not receive further cooking by the processor, such as a seafood salad. In these cases, the ingredient receiving and storage steps may also require time/temperature controls and be designated as CCPs (unless the ingredient is received and stored frozen). If these ingredients are to be used in a product that will be heated sufficient to kill any pathogens that may be present, these processing steps may not need to be designated as CCPs. However, in making this determination, you should consider the potential for Staphylococcus aureus or Bacillus cereus toxin formation. Remember that these toxins are not likely to be inactivated by heat.
Time/temperature controls may be required at the following steps (CCPs):
- Receiving;
- Cooling after cooking;
- Processing after cooking, such as:
- Slicing hot-smoked salmon;
- Mixing seafood salad;
- Picking crabmeat;
- Packaging;
- In-process and finished product refrigerated (not frozen) storage.
Time/temperature controls will usually not be needed at processing steps that meet the following conditions:
- Continuous, mechanical processing steps that are
brief, such as:
- Mechanical size grading of cooked shrimp;
- Mechanical forming of surimi-based analog products;
- Individual quick freezing;
- Processing steps that are brief and unlikely to
contribute significantly to the cumulative time/
temperature exposure to unrefrigerated
conditions, such as:
- Date code stamping;
- Case packing;
- Processing steps where the product is held in a
frozen state, such as:
- Glazing;
- Assembly of orders for distribution;
- Frozen product storage;
- Processing steps where the product is held at
temperatures above 140°F, such as:
- Initial stage of cooling;
- Hot holding.
In the processing of many food products, especially products that contain meat or rice, rapid cooling after cooking is important to the safety of the product. This is the case for two reasons. First, spore-forming pathogens, such as Clostridium perfringens and Bacillus cereus, may survive the cooking process and grow and/or produce toxin in the product during cooling and subsequent handling. In fact, the heat from the cooking process may actually initiate growth of the surviving spores. Second, the cooked product may be recontaminated with pathogens after cooking. Because the normally-occurring spoilage organisms are no longer present to compete with the pathogens in cooked product, rapid growth and toxin formation by the pathogens may be possible.
In deciding whether the cooling step after cooking is significant in your product, consider the following. Some cooking processes, such as the retort cooking of blue crabs (typical of the East coast processing technique) may be adequate to kill even the spores of C. perfringens and B. cereus. In some processes cooling is performed: 1) before any significant handling of the cooked product; and 2) in the same container in which the product was cooked. Again, this technique is typical of East coast retort processing of blue crab. Under these conditions cooling after cooking may not need to be identified as a critical control point for this hazard. However, such a determination is dependent upon strict adherence to good sanitation practices, to further minimize the risk of recontamination with pathogens.
When significant handling occurs before or during the cooling process, when the cooked product comes into contact with equipment that was not heated along with the product, or when the cooking process is not adequate to kill the spores of C. perfringens and B. cereus, cooling after cooking may need to be identified as a critical control point for this hazard.
Raw, ready-to-eat
These products are not heated during processing to a temperature that will kill pathogens. They are often consumed without cooking. Examples include: cold-smoked fish and raw oysters, clams, and mussels.
Like cooked, ready-to-eat products, raw ready-to-eat products may develop pathogen hazards as a result of cross contamination and growth. They may also contain pathogens that were present in the raw material, and which are capable of growth in the finished product. For example, oysters harvested during the warm weather months may contain Vibrio vulnificus or Vibrio parahaemolyticus, bacterial pathogens which are capable of growth in the raw product.
Time/temperature controls may be required at the following processing steps (CCPs):
- Receiving;
- Processing, such as:
- Shucking;
- Portioning;
- Packaging;
- Raw material, in-process, and finished product storage.
Time/temperature controls will usually not be needed at processing steps that meet the following conditions:
- Continuous, mechanical processing steps that are brief, such as mechanical filleting;
- Processing steps that are brief and unlikely to
contribute significantly to the cumulative time/
temperature exposure to unrefrigerated
conditions, such as:
- Date code stamping;
- Case packing;
- Processing steps where the product is held in a
frozen state, such as:
- Assembly of orders for distribution;
- Frozen storage.
Proceed to Step #13 (Chapter 2) or to Step #10 of the next potential hazard.
HACCP Plan Form
STEP #14: Set the critical limits (CL).
For each processing step where "pathogen growth and toxin formation as a result of time/temperature abuse" is identified as a significant hazard on the HACCP Plan Form identify the maximum or minimum value to which a feature of the process must be controlled in order to control the hazard.
You should set the CL at the point that if not met the safety of the product may be questionable. If you set a more restrictive CL you could, as a result, be required to take corrective action when no safety concern actually exists. On the other hand, if you set a CL that is too loose you could, as a result, allow unsafe product to reach the consumer.
As a practical matter it may be advisable to set an operating limit that is more restrictive than the CL. In this way you can adjust the process when the operating limit is triggered, but before a triggering of the CL would require you to take corrective action. You should set operating limits based on your experience with the variability of your operation and with the closeness of typical operating values to the CL.
Following is some general guidance on setting critical limits for the control strategy example discussed in Step #12. More specific guidance follows.
• Control Strategy Example 1 - Time/temperature control
Critical Limit: A combination of product internal temperatures and times that will prevent growth of target pathogens to unsafe levels and/or will prevent toxin formation;
AND/OR
A combination of ambient (e.g. air, water, or brine) temperatures and times of exposure that will prevent growth of target pathogens to unsafe levels and/or will prevent toxin formation;
AND/OR
The presence of sufficient cooling media to achieve either of the above purposes (e.g. adequate ice to completely surround the product);
AND/OR
Limits for critical aspects of the process that affect the rate of cooling, as established by a cooling rate study (e.g. volume or size of product being cooled).
Refer to the data provided in Table #A-2 (Appendix 4) for assistance in establishing appropriate cumulative time/temperature exposure critical limits for the pathogens that are significant hazards in your product. The critical limits described are intended to keep the pathogens from reaching the rapid growth phase (i.e. keep them in the lag phase). In summary, the table indicates that:
- If the product is held at internal temperatures above 70°F (21.1°C) during processing, exposure time should ordinarily be limited to two hours (three hours if Staphylococcus aureus is the only pathogen of concern);
- If the product is held at internal temperatures above 50°F (10°C), but not above 70°F (21.1°C), exposure time should ordinarily be limited to six hours (twelve hours if Staphylococcus aureus is the only pathogen of concern);
- If the product is held at internal temperatures both above and below 70°F (21.1°C), exposure times above 50°F (10°C) should ordinarily be limited to 4 hours, as long as no more than 2 of those hours are above 70°F (21.1°C).
Keep in mind that pathogen growth is relatively slow at temperatures below 70°F (21.1°C). In most cases growth is very slow below 50°F (10°C), and 40°F (4.4°C) is below the minimum growth temperature of most pathogens, although there are some exceptions. On the other hand, pathogens grow relatively fast at temperatures above 70°F (21.1°C).
FIGURE 12-1: Internal Temperature
Profile -- Blue Crabmeat Processing
Partial Cooling Only After Cook With Significant Handling
Before Full Cooling
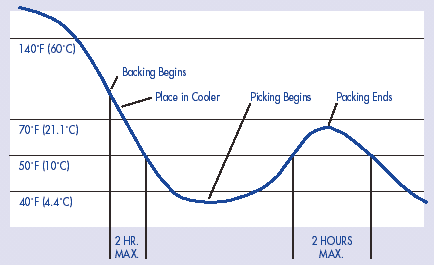
FIGURE 12-2: Internal Temperature
Profile -- Blue Crabmeat Processing
Cooling After Cook in Original Container With No
Significant Handling During Cooling
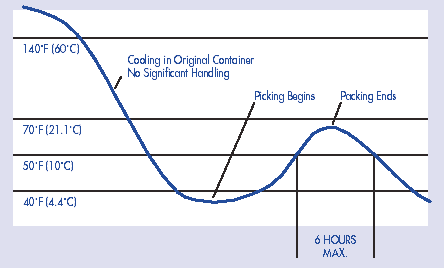
The time/temperature relationships in the table are designed to refer to the time that your product is held at a particular internal product temperature. You may need to study temperature fluctuations in your product under normal operating conditions in order to relate the values in the table to cumulative time or exposure to unrefrigerated conditions. Drawing a graph depicting the time/temperature profile throughout your processing may help you in calculating the cumulative time/temperature exposure of your product. Figures 12-1 and 12-2 are examples of time/temperature profiles for crabmeat processing. Remember that the values provided in Table A-2 (Appendix 4) are cumulative exposure throughout processing.
For product-specific calculations you may choose to use predictive microbiology models, such as the U.S.D.A. Pathogen Modeling Program (PMP) or the United Kingdom's Food MicroModel (FMM). However, validating the reliability of predictions from such models for your food is essential.
Finished product storage critical limits should be based on the minimum growth temperatures of the pathogens of concern. You should establish a maximum storage temperature that will control pathogen growth and toxin formation throughout the shelf life of your product. It is not always necessary or practical to establish a maximum storage temperature that is below the minimum growth temperature of all of the pathogens of concern. A maximum storage temperature of 40°F (4.4°C) is often selected and is generally safe for most refrigerated, microbiologically sensitive products. However, where refrigeration is necessary to control the growth of nonproteolytic Clostridium botulinum, a maximum storage temperature of 38°F (3.3°C) is usually appropriate (see Chapter 13 for additional information). You should consider the same factors when you set critical limits for raw material and in-process refrigerated product storage.
Cooked, ready-to-eat products provide an additional complication. Survival of most pathogens through a cook step is unlikely if proper controls are used (see Chapter 16). Therefore, cooling after cooking that occurs before the product receives any further significant handling, or contacts any processing equipment that was not heated along with the product, need not be considered as part of the cumulative time/temperature exposure. It is advisable to fully cool product before it is further handled, in order to minimize pathogen growth and toxin formation. However, if significant handling does take place before cooling is completed, the cumulative time/temperature exposure to unrefrigerated conditions (described earlier) should be calculated from the time that the product is first handled after cooking.
If you identified cooling after cooking as a critical control point for this hazard in Step #13 (e.g., because of the potential for Clostridium perfringens or Bacillus cereus growth or toxin formation, the product should generally be cooled from 140°F (60°C) to 70°F (21.1°C) or below within two hours and to 40°F (4.4°C) or below within another four hours. The cooling rate critical limit is separate from the cumulative time/temperature critical limit described earlier.
Based on the type of monitoring that will be performed, it may be more convenient to state critical limits as a maximum time, a maximum temperature, or a combination of time and temperature. Generally, a critical limit that combines time and temperature is superior because it more closely approximates the actual growth characteristics of pathogens. If a critical limit references a temperature only, the temperature should ordinarily be at or near the minimum growth temperature of the target pathogen. If the critical limit references a time only, the time should ordinarily represent a safe exposure time for the target pathogen under the worst conditions that are reasonably likely to occur (i.e. nearest its optimum growth temperature).
Example:
A crab meat processor (retort process) identifies a series of post-cook processing and storage steps (e.g. backing, picking, packing, and refrigerated storage) as critical control points for pathogen growth and toxin formation. The product is packaged in a plastic container with a snap lid (aerobic). This minimizes the risk of Clostridium botulinum and Clostridium perfringens growth. However, the potential exists for the other pathogens listed in Table #A-1 (Appendix 4) to be present and to grow, because neither the water activity, acidity, or salt content of the food will inhibit them. Initial cooling takes place in the cooking crates. The product may not be fully cooled before handling. The processor sets the following critical limits:
- For the finished product cooler: a maximum cooler temperature of 40° F (4.4° C);
- For backing, picking, and packing: a maximum cumulative time of 2 hours at product internal temperatures above 50°F (10°C), starting when the cooked crabs are first handled. Alternatively, the processor could set a critical limit of no more than 4 hours at product internal temperatures above 50°F (10°C), no more than 2 of which are above 70°F (21.1°). These limits are necessary because the crabs are handled while still warm (e.g. above 70°F [21.1°C]). Cooling that takes place after the product is handled is included in the limit.
Example:
Another crab meat processor also identifies a series of post-cook processing and storage steps (e.g. backing, picking, packing, and refrigerated storage) as critical control points. The product is packaged in the same way. However, this product is cooled fully before handling and ice is used on the product during processing to control time/temperature abuse. The processor sets the following critical limits:
- For the finished product cooler: sufficient ice to fully cover the containers at all times;
- For backing, picking, and packing: a maximum product temperature of 50° F (10° C) at all times. Specifying a time of exposure is not necessary in this case, because it is not reasonably likely that the product would be held long enough that significant pathogen growth could occur at this temperature (e.g. 2 to 21 days) depending upon the pathogen.
Enter the critical limit(s) in Column 3 of the HACCP Plan Form.
STEP #15: Establish monitoring procedures.
For each processing step where "pathogen growth and toxin formation as a result of time/temperature abuse" is identified as a significant hazard on the HACCP Plan Form, describe monitoring procedures that will ensure that the critical limits are consistently met.
To fully describe your monitoring program you should answer four questions: 1) What will be monitored? 2) How will it be monitored? 3) How often will it be monitored (frequency)? 4) Who will perform the monitoring?
It is important for you to keep in mind that the feature of the process that you monitor and the method of monitoring should enable you to determine whether the CL is being met. That is, the monitoring process should directly measure the feature for which you have established a CL.
You should monitor often enough so that the normal variability in the values you are measuring will be detected. This is especially true if these values are typically close to the CL. Additionally, the greater the time span between measurements the more product you are putting at risk should a measurement show that a CL has been violated.
Following is guidance on establishing monitoring procedures for the control strategy example discussed in Step #12. Note that the monitoring frequencies that are provided are intended to be considered as minimum recommendations, and may not be adequate in all cases.
What Will Be Monitored?
• Control Strategy Example 1 - Time/temperature control
For receiving of refrigerated (not frozen) cooked, ready-to-eat or raw, ready-to-eat fishery products to be stored, or processed without further cooking:
What: The internal temperature of the fishery product throughout transportation;
OR
The temperature of the truck or other carrier throughout transportation;
OR
For fishery products with a transit time of four hours or less: The internal temperature of a representative number of containers in the lot at time of delivery;
OR
The adequacy of ice or chemical cooling media at time of delivery.
For raw material, in-process, or finished product refrigerated storage or for refrigerated processing:
What: The temperature of the cooler or refrigerated processing area.
For raw material, in-process, or finished product storage under ice or chemical cooling media:
What: The adequacy of ice or chemical cooling media.
For cooling after cooking:
What: The internal temperature of the product, and the length of time between the end of the cook (or the time that the product internal temperature fell below 140°F [60°C]) and the time that measurement was made;
OR
The critical aspects of the process that affect the rate of cooling, as established by a cooling rate study (e.g. product internal temperature at the start of cooling, cooler temperature, quantity of ice, quantity or size of product being cooled).
For unrefrigerated processing and packaging:
What: The length of time of exposure of the product to unrefrigerated conditions, and either the internal temperature of the product or the ambient temperature;
OR
The length of time of exposure of the product to unrefrigerated conditions when the critical limit assumes a temperature greater than 70°F (21.1°C);
OR
The length of time of exposure of the product to unrefrigerated conditions when a study demonstrates that under ordinary conditions product does not exceed 70°F (21.1°C) when exposed for the length of time specified by the critical limits and that time/temperature combination is adequate to control the growth of the pathogens of concern;
OR
The internal temperature of the product (where temperatures are held below a temperature at which growth is minimized [e.g. 50°F (10°C) for Salmonella spp.] or held above 140°F [60°C] during processing);
OR
The ambient air temperature (where ambient air temperature is low enough to control microbial growth [e.g. 50°F (10°C) for Salmonella spp.]).
How Will Monitoring Be Done?
• Control Strategy Example 1 - Time/temperature control
For receiving of refrigerated (not frozen) cooked, ready-to-eat or raw, ready-to-eat products to be stored, or processed without further cooking:
How: Use a time/temperature integrator for product internal temperature monitoring during transit;
OR
Use a maximum indicating thermometer for ambient air temperature monitoring during transit;
OR
Use a digital time/temperature data logger for product internal temperature or ambient air temperature monitoring during transit;
OR
Use a recorder thermometer for ambient air temperature monitoring during transit;
OR
Use a dial or digital thermometer for internal product temperature monitoring at receipt;
OR
Make visual observations of the adequacy of ice or other cooling media in a sufficient number of containers to represent all of the product.
For raw material, in-process, or finished product refrigerated storage or for refrigerated processing:
How: Use a digital time/temperature data logger;
OR
Use a recorder thermometer;
OR
Use a high temperature alarm with 24-hour monitoring.
For raw material, in-process, or finished product storage under ice or chemical cooling media:
How: Make visual observations of the adequacy of ice or chemical cooling media in a sufficient number of containers to represent all of the product.
For cooling after cooking:
How: Use a dial or digital thermometer and visual check on time of cooling;
OR
Use a digital time/temperature data logger;
OR
Use appropriate instruments (e.g. dial thermometer, digital time/temperature data logger) and/or visual observations as necessary to measure the critical aspects of the process that affect the rate of cooling, as established by a cooling rate study.
Example:
A crayfish processor has identified cooling after the cook step as a critical control point for pathogen growth and toxin formation. The processor established a cooling critical limit of no more than two hours from 140° F (60° C) to 70°F (21.1°C) and no more than four more hours from 70° F (21.1° C) to 40° F (4.4° C). The processor uses marked batches of cooked product to monitor the cooling process. The time that the marked batch is removed from the cooker is monitored visually and the internal temperature of the product in that batch two hours after cooking and four more hours after cooking is monitored with a dial thermometer.Example:
Another crayfish processor has similarly identified cooling as a critical control point and has established the same critical limit. The processor uses a digital time/temperature data logger to monitor the cooling rate of the cooked product.Example:
Another crayfish processor has similarly identified cooling as a critical control point. This processor has performed a cooling rate study that determined that a cooling rate of no more than two hours from 140°F (60° C) to 70° F (21.1° C) and no more than four more hours from 70° F (21.1° C) to 40° F (4.4° C) can be achieved as long as certain conditions are met in the cooling process. The study determined that the following critical limits must be met: a cooler temperature of no more than 60°F (15.6° C) during the first two hours of cooling and no more than 40° F (4.4° C) during the remainder of cooling; and, no more than 1000 lbs of crayfish in the cooler. The processor monitors the cooler temperature with a recorder thermometer and monitors the weight of product at receiving with a scale.
For unrefrigerated processing and packaging:
How: Use a dial or digital thermometer for product or ambient air temperature;
AND/OR
Make visual observations of length of exposure to unrefrigerated conditions.
Example:
A crab meat processor has identified a series of processing steps (e.g. backing, picking, and packing) as critical control points for pathogen growth. The processor established a critical limit of no more than two cumulative hours of exposure to unrefrigerated temperature during these processing steps. The processor uses marked product containers to monitor the progress of the product through the three processing steps. The time that the marked container is removed from and returned to refrigeration is monitored visually.Example:
Another crabmeat processor with identical CCPs, has established a more complex set of critical limits -no more than two cumulative hours with product internal temperatures above 70°F (21.1°C), and no more than six cumulative hours with product internal temperatures above 50° F (10° C). This processor also uses marked containers to monitor the progress of the product through the process. However, in addition to monitoring time, the processor also monitors product internal temperature for the marked containers. This monitoring technique provides the processor more flexibility in processing but requires more monitoring effort.Example:
Another crabmeat processor that fully cools the product before handling has identified the same CCPs. The processor has determined through study that, under ordinary circumstances, in 3 1/2 hours of exposure to ambient (room) temperature the product will remain below 70°F (21.1°C). The processor has set a critical limit of 3 1/2 hours out of refrigeration. The processor monitors visually the time that picking begins after each batch of crabs is brought into the processing room and the time that the last of the containers of crabmeat from this batch has been placed on ice.Example:
A lobster meat processor has identified the meat removal process as a critical control point for pathogen growth. The operation is performed under near refrigeration conditions (50°F [10°C]). The processor has determined that exposure time sufficient to jeopardize the safety of the product at these temperatures is not reasonably likely to occur. The processor only monitors ambient air temperature with a digital data logger.
How Often Will Monitoring Be Done (Frequency)?
• Control Strategy Example 1 - Time/temperature control
For receiving of refrigerated (not frozen) cooked, ready-to-eat or raw, ready-to-eat products to be stored, or processed without further cooking:
Frequency: Each shipment.
For raw material, in-process, or finished product refrigerated storage or for refrigerated processing:
Frequency: Continuous monitoring by the instrument itself, with visual check of the instrument at least once per day.
For raw material, in-process, or finished product storage under ice or chemical cooling media:
Frequency: At least twice per day;
OR
For finished product storage, at least immediately prior to shipment.
For cooling after cooking:
Frequency: At least every two hours;
OR
For critical aspects of the cooling process, as often as necessary to ensure control of the process.
For unrefrigerated processing and packaging:
Frequency: At least every two hours;
OR
Each batch.
Who Will Perform the Monitoring??
• Control Strategy Example 1 - Time/temperature control
Who: With recorder thermometers, time/temperature integrators, high temperature alarms, maximum indicating thermometers, and digital data loggers, monitoring is performed by the equipment itself. However, anytime that such instrumentation is used, a visual check should be made at least once per day in order to ensure that the critical limits have consistently been met. These checks, as well as dial thermometer checks, time of expo sure checks, and adequacy of ice or other cooling media checks may be performed by the receiving employee, the equipment operator, a production supervisor, a member of the quality control staff, or any other person who has an understanding of the process and the monitoring procedure.
Enter the "What," "How," "Frequency," and "Who" monitoring information in Columns 4, 5, 6, and 7, respectively, of the HACCP Plan Form.
STEP #16: Establish corrective action procedures.
For each processing step where "pathogen growth and toxin formation as a result of time/temperature abuse" is identified as a significant hazard on the HACCP Plan Form, describe the procedures that you will use when your monitoring indicates that the CL has not been met.
These procedures should: 1) ensure that unsafe product does not reach the consumer; and, 2) correct the problem that caused the CL deviation. Remember that deviations from operating limits do not need to result in formal corrective actions.
Following is guidance on establishing corrective action procedures for the control strategy example discussed in Step #12.
• Control Strategy Example 1 - Time/temperature control
For receiving of refrigerated (not frozen) cooked, ready-to-eat or raw, ready-to-eat products to be stored, or processed without further cooking:
Corrective Action: Reject shipment, if the CL is not met;
OR
Hold the product until it can be evaluated based on its total time/temperature exposure;
AND
Discontinue use of supplier or carrier until evidence is obtained that transportation practices have changed.
Note: If an incoming lot that fails to meet a receiving critical limit is mistakenly accepted, and the error is later detected, the following actions should be taken: 1) the lot and any products processed from that lot should be destroyed, diverted to a nonfood use or to a use in which the critical limit is not applicable, or placed on hold until a food safety evaluation can be completed; and 2) any products processed from that lot that have already been distributed should be recalled and subjected to the actions described above.
For other critical control points:
Corrective Action: Take one or several of the following actions as necessary to regain control over the operation after a CL deviation:
- Add ice to the affected product;
OR
- Make repairs or adjustments to the malfunctioning
cooler;
OR
- Move some or all of the product in the
malfunctioning cooler to another cooler;
OR
- Return the affected in-process product to the
cooler;
OR
- Freeze the affected product;
OR
- Modify the process as needed to reduce the time/temperature exposure;
AND
Take one of the following actions to product involved in the critical limit deviation:
- Destroy the product;
OR
- Hold the product until it can be evaluated based
on its total time/temperature exposure;
OR
- Cook or recook the product. In this case, special
attention must be paid to the fact that any Staphylococcus
aureus or Bacillus cereus toxin that
may be present may not be inactivated by heat;
OR
- Divert the product to a use in which the critical
limit is not applicable (e.g. divert crabmeat to
a stuffed flounder operation). In this case,
special attention must be paid to the fact that
any Staphylococcus aureus or Bacillus
cereus toxin that may be present may not be
inactivated by heat;
OR
- Divert the product to a non-food use.
Enter the corrective action procedures in Column 8 of the HACCP Plan Form.
STEP #17: Establish a recordkeeping system.
For each processing step where "pathogen growth and toxin formation as a result of time/temperature abuse" is identified as a significant hazard on the HACCP Plan Form, list the records that will be used to document the accomplishment of the monitoring procedures discussed in Step #15. The records should clearly demonstrate that the monitoring procedures have been followed, and should contain the actual values and observations obtained during monitoring.
Following is guidance on establishing a recordkeeping system for the control strategy example discussed in Step #12.
• Control Strategy Example 1 - Time/temperature control
For receiving of refrigerated (not frozen) cooked, ready-to-eat or raw, ready-to-eat products to be stored or processed without further cooking:
Records: Receiving record showing the results of the time/temperature integrator checks;
OR
Printout from digital time/temperature data logger;
OR
Recorder thermometer chart;
OR
Receiving record showing the results of the maximum indicating thermometer checks;
OR
The results of internal product temperature monitoring at receipt;
AND
The date and time of departure and arrival of the vehicle;
OR
Receiving record showing the results of the ice or other cooling media checks.
For raw material, in-process, or finished product refrigerated storage or refrigerated processing:
Records: Printout from digital time/temperature data logger;
OR
Recorder thermometer chart;
OR
Storage record showing the results of the high temperature alarm checks.
For raw material, in-process, or finished product storage under ice or chemical cooling media:
Records: Storage record showing the results of the ice or other cooling media checks.
For cooling after cooking:
Records: Processing record showing the results of the time/temperature checks;
OR
Printout from digital time/temperature data logger;
OR
Appropriate records (e.g. processing record showing the results of the time and temperature checks and/or volume of product in cooler, printout from digital time/temperature data logger) as necessary to document the monitoring of the critical aspects of the process that affect the rate of cooling, as established by a cooling rate study.
For unrefrigerated processing and packaging:
Records: Processing records showing the results of time and/or temperature checks;
OR
Printout from digital time/temperature data logger.
Enter the names of the HACCP records in Column 9 of the HACCP Plan Form.
STEP #18: Establish verification procedures.
For each processing step where "pathogen growth and toxin formation as a result of time/temperature abuse" is identified as a significant hazard on the HACCP Plan Form, establish verification procedures that will ensure that the HACCP plan is: 1) adequate to address the hazard; and, 2) consistently being followed.
Following is guidance on establishing verification procedures for the control strategy example discussed in Step #12.
• Control Strategy Example 1 - Time/temperature controlVerification: Review monitoring, corrective action, and verification records within one week of preparation;
AND
When digital time/temperature data loggers, recorder thermometers, or high temperature alarms are used for in-plant monitoring, check for accuracy against a known accurate thermometer (NIST-traceable) at least once per day;
AND
When digital time/temperature data loggers or recorder thermometers are used for monitoring of transport conditions at receiving, check for accuracy against a known accurate thermometer (NIST-traceable). Verification should be conducted on new suppliers' vehicles and at least quarterly for each supplier thereafter. Additional verifications may be warranted based on observations at receipt (e.g. refrigeration units appear to be in poor repair, or readings appear to be erroneous);
AND
When visual checks of ice or cooling media are used to monitor the adequacy of coolant, periodically measure internal temperatures of fish to ensure that the ice or cooling media is sufficient to maintain product temperatures at 40°F (4.4°C) or less;
AND
When dial or digital thermometers or maximum indicating thermometers are used for monitoring, check for accuracy against a known accurate thermometer (NIST-traceable) when first used and at least once per year thereafter. (Note: optimal calibration frequency is dependent upon the type, condition, and past performance of the monitoring instrument.)
Enter the verification procedures in Column 10 of the HACCP Plan Form.
TABLE 12-1Control Strategy Example 1 - Time/temperature control - Version 1 This table is an example of a portion of a HACCP plan relating to the control of pathogen growth and toxin formation as a result of time/temperature abuse for a processor of blue crabmeat (typical of Gulf Coast boiling processing method), using Control Strategy Example 1 - Time/temperature control. It is provided for illustrative purposes only. Pathogen growth and toxin formation may be only one of several significant hazards for this product. Refer to Tables 3-1, 3-2, and 3-3 (Chapter 3) for other potential hazards (e.g. Chemical contaminants, pathogen survival through cooking, and metal fragments). Example Only | |||||||||
| (1) | (2) | (3) | (4) | (5) | (6) | (7) | (8) | (9) | (10) |
| Critical Control Point (CCP) | Significant Hazard(s) | Critical Limits for each Preventive Measure | Monitoring | Corrective Action(s) | Records | Verification | |||
|---|---|---|---|---|---|---|---|---|---|
| What | How | Frequency | Who | ||||||
| Backing | Pathogen growth and toxin formation | No more than 2 hrs. cumulative
time during backing, picking and packing. Note: This CL is necessary because the crabs are handled at internal temperatures above 70°F during backing |
Time of product exposure to unrefrigerated conditions | Visual observation of marked containers | Start marked container appx. every two hours during backing | Production supervisor | Immediately ice product or move
to cooler Hold and evaluate based on total time/temperature exposure |
Production record | Review monitoring and corrective action records within one week of preparation |
| Backed crab cooler | Pathogen growth and toxin formation | Cooler maintained at or below 40°F | Cooler temperature | Digital time/temperature data logger | Continuous with visual check once per day | Production supervisor | Move to alternate cooler and/or
add ice Hold and evaluate based on total time/temperature exposure |
Data logger printout | Check accuracy of data logger
once per day; Review monitoring, corrective action, and verification records within one week of preparation |
| Picking | Pathogen growth and toxin formation | No more than 2 hrs. cumulative time during backing, picking, and packing | Time of product exposure to unrefrigerated conditions | Visual observation of marked containers | Start marked container appx. every two hours during picking | Production supervisor | Immediately ice product or move
to cooler Hold and evaluate based on total time/temperature exposure |
Production record | Review monitoring and corrective action records within one week of preparation |
| Packing | Pathogen growth and toxin formation | No more than 2 hrs. cumulative time during backing, picking, and packing | Time of product exposure to unrefrigerated conditions | Visual observation of marked containers | Start marked container appx. every two hours during picking | Production supervisor | Immediately ice product or move
to cooler Hold and evaluate based on total time/temperature exposure |
Production record | Review monitoring and corrective action records within one week of preparation |
| Finished product cooler | Pathogen growth and toxin formation | Cooler maintained at or below 40°F | Cooler temperature | Digital time/temperature data logger | Continuous with visual check once per day | Production employee | Move to alternate cooler and/or
add ice Hold and evaluate based on total time/temperature exposure |
Data logger printout | Check accuracy of data logger
once per day Review monitoring, corrective action, and verification records within one week of preparation |
TABLE 12-2Control Strategy Example 1 - Time/temperature control - Version 2 This table is an example of a portion of a HACCP plan relating to the control of pathogen growth and toxin formation as a result of time/temperature abuse for a processor of blue crabmeat (typical of East coast retort processing method), using Control Strategy Example 1 - Time/temperature control. It is provided for illustrative purposes only. Pathogen growth and toxin formation may be only one of several significant hazards for this product. Refer to Tables 3-1, 3-2, and 3-3 (Chapter 3) for other potential hazards (e.g. Chemical contaminants, pathogen survival through cooking, and metal fragments). Example Only | |||||||||
| (1) | (2) | (3) | (4) | (5) | (6) | (7) | (8) | (9) | (10) |
| Critical Control Point (CCP) | Significant Hazard(s) | Critical Limits for each Preventive Measure | Monitoring | Corrective Action(s) | Records | Verification | |||
|---|---|---|---|---|---|---|---|---|---|
| What | How | Frequency | Who | ||||||
| Cooked crab cooler | Pathogen growth and
toxin formation Note: Control is necessary at this step because the processor has not established that the cook step is adequate to kill the spores of Clostridium perfringens or Bacillus cereus |
Crabs cooled from 140°F to 70°F in 2 hrs and 70°F to 40°F in 4 more hrs. | Cooked crab internal temperature | Dial thermometer in marked batches of cooked crabs | Start marked batch approx. every two hours during cooking | Production supervisor | Move part of load to alternate
cooler and/or add ice Hold and evaluate based on total time/temperature exposure |
Production record | Check accuracy of data logger
once per day; Check accuracy of digital thermometer once per day; |
| Cooler maintained at or below 40°F after cooling completed | Cooler temperature | Digital time/ temperature data logger | Continuous with visual check once per day | Production supervisor | Same | Data logger printout | Review monitoring, corrective action and verification records within one week of preparation | ||
| Picking/ boning/ packing | Pathogen growth and toxin formation | No more than 3 1/2 hours cumulative time during picking, boning, and packing (beginning when cooked crabs are first handled in picking room) Note: This critical limit is based on a study that demonstrates that, under ordinary circumstances, the product does not exceed 70°F in 3 1/2 hours exposure to ambient temperature | Time of product exposure to unrefrigerated conditions | Visual observation of time that picking begins for each batch of cooked crabs that is brought into the picking room | Every Batch | Picking room supervisor | Pasteurize or freeze
the product Hold and evaluate based on total time/temperature exposure |
Cooked crab record | Review monitoring
and corrective action records within one week of
preparation Study showing temperature profile of product during processing |
| Visual observaton of time that the last container of crabmeat from the batch is packed on ice | Every batch | Packing room employee | Packing record | ||||||
| Finished product storage | Pathogen growth and toxin formation | Finished product containers completely surrounded with ice | Adequacy of ice | Visual observation | Each case immediately before shipping | Shipping employee | Re-ice Hold and evaluate based on total time/temperature exposure |
Shipping record | Review monitoring and corrective action records within one week of preparation |
See also:
Foodborne Pathogenic Microorganisms and Natural Toxins Handbook (Bad Bug Book)
Seafood Information and Resources
Seafood HACCP | Fish & Fisheries Products Hazards & Controls Guidance: 3rd Edition (2001)
Foods Home | FDA Home | Search/Subject Index | Disclaimers & Privacy Policy | Accessibility/Help
Hypertext updated by dav/kwg/dms 2002-JUN-14