Chapter 9: Aerobic Plate Count
Updated: 9/20/00
Contents
Potential Food Safety Hazard
The aerobic plate count (APC) indicates the level of microorganisms in a product
(Maturin and Peeler, 1998). Aerobic plate counts on fish and fishery products generally do
not relate to food safety hazards, but sometimes can be useful to indicate quality, shelf
life and post heat-processing contamination. Fresh fish and fishery products often have an
APC of 104-105/g, although there are examples of seafoods with an
APC of 106-108/g without objectionable quality changes (Nickelson
and Finne, 1992).
The plating medium (nutrient source) used in an APC can affect the number and types of
bacteria isolated because of differences in nutrient and salt requirements of the various
microorganisms. For many fish and fishery products, a plate incubation temperature of
25ºC (77ºF) produces significantly higher numbers of bacteria than incubation at 35ºC
(95ºF) (Nickelson and Finne, 1992).
Contents
FDA Guidelines
Table 9-1. FDA guidelines for APC in fish and fishery products.
| Product |
Guideline |
Reference |
| Raw breaded shrimp |
Aerobic plate counts (35ºC
[95ºF]) – The mean log of 16 units of finished product breaded shrimp collected
prior to freezing is greater than 5.00 (i.e., geometric mean greater than 100,000/g) and
exceeds the mean log of 16 units of stock shrimp by more than twice the standard error of
their difference (2 SED) |
FDA, 1996a |
| Clams oysters, and mussels, fresh
or frozen--domestic |
APC - 1 or more of 5 subs exceeding
1,500,000/g or 2 or more exceeding 500,000/g |
FDA, 1998 |
| Clams, oysters, and mussels fresh
or frozen--imports |
APC - 500,000/g (average of subs
of 3 or more of 5 subs) |
FDA, 1998 |
Contents
State Guidelines
Table 9-2. State APC Guidelines.
| State |
Product |
Maximum APC |
| Alabama |
Oysters, fresh or frozen |
5 ´ 105/g |
| Alaska |
Oysters, clams, and mussels |
5 ´ 105/g |
| Oysters, clams, and mussels: in shell or
shucked, but not eviscerated |
5 ´ 105/g |
| Oysters, clams, and mussels: eviscerated |
1 ´ 105/g |
| Arizona |
Clams, mussels, and oysters |
5 ´ 105/g |
| Arkansas |
- |
|
| California |
Oysters, clams, and mussels |
5 ´ 105/g |
| Colorado |
Oysters, clams, mussels, and scallops |
5 ´ 105/g |
| Connecticut |
Oysters, clams, and mussels |
5 ´ 105/g |
| Delaware |
Clams, mussels, or other mollusks, fresh or
frozen |
5 ´ 105/g |
| Florida |
Blue crab |
105/g |
| Shellfish |
5 ´ 105/g |
| Georgia |
Clams, mussels, and oysters - fresh or frozen |
5 ´ 105/g |
| Fried clams, frozen |
104/g |
| Scallops, fried, frozen |
104/g |
| Scallops, breaded, frozen |
104/g |
| Crabmeat, fresh cooked |
104/g |
| Deviled crab, frozen, cooked |
104/g |
| Deviled crab, fresh, uncooked |
106/g |
| Shrimp, peeled, cooked |
105/g |
| Shrimp, breaded, frozen, raw |
106/g |
| Fish, frozen, breaded, fried |
2.5 ´ 104/g |
| Fish, frozen, breaded, raw |
105/g |
| Fried fish cakes, frozen |
104/g |
| Hawaii |
Oysters, clams, mussels, fresh or frozen |
5 ´ 105/g |
| Idaho |
- |
- |
| Illinois |
- |
- |
| Indiana |
- |
- |
| Iowa |
- |
- |
| Kansas |
- |
- |
| Kentucky |
Oysters, clams, scallops, shrimp, fresh or
frozen |
5 ´ 105/g |
| Louisiana |
- |
- |
| Maine |
- |
- |
| Maryland |
Fresh crabmeat |
105/g |
| Pasteurized crabmeat |
2.5 ´ 104/g |
| Oysters, clams, mussels, fresh or frozen |
5 ´ 105/g |
| Massachusetts |
Oysters, clams, mussels, fresh or frozen |
5 ´ 105/ml |
| Michigan |
- |
- |
| Minnesota |
- |
- |
| Mississippi |
Oysters, clams, mussels, fresh or frozen |
5 ´ 105/g |
| Missouri |
Oysters, clams, mussels, fresh or frozen |
5 ´ 105/100ml |
| Foods |
1.5 ´ 106/g |
| Montana |
- |
- |
| Nebraska |
Oysters, clams, mussels, fresh or frozen |
5 ´ 105/g |
| Deli foods (shrimp salad, etc.) |
105/g |
| Nevada |
- |
- |
| New Hampshire |
Oysters, softshell clams, fresh or frozen |
5 ´ 105/g |
| New Jersey |
Oysters, clams, mussels, fresh or frozen |
5 ´ 105/g |
| "Potentially hazardous" (tuna,
shrimp salad) |
104/g |
| New Mexico |
- |
- |
| New York |
- |
- |
| North Carolina |
Shellfish |
5 ´ 105/g |
| Crustacea, fresh |
104/g |
| Crustacea, pasteurized |
3 ´ 103/g |
| North Dakota |
- |
- |
| Ohio |
- |
- |
| Oklahoma |
- |
- |
| Oregon |
Oysters, clams, mussels, fresh or frozen |
5 ´ 105/100g |
| Pennsylvania |
- |
- |
| Rhode Island |
Oysters, clams, mussels, fresh or frozen |
5 ´ 105/g |
| Fresh seafood |
106/g |
| Smoked fish |
105/g |
| South Carolina |
Fresh cooked blue crabmeat |
105/g |
| Pasteurized blue crabmeat |
2.5 x 104/g |
| Oysters, clams, mussels, fresh or
frozen |
5 ´ 105/g |
| South Dakota |
- |
- |
| Tennessee |
- |
- |
| Texas |
Crabmeat |
105/g |
| Oysters, clams, mussels, fresh or
frozen |
5 ´ 105/g |
| Utah |
- |
- |
| Vermont |
- |
- |
| Virginia |
Fresh blue crabmeat |
105/g |
| Pasteurized blue crabmeat |
3 ´ 103/g |
| Shellfish - shucked or in the shell |
5 ´ 105/g |
| Washington |
Molluscan shellfish (Oysters, clams, mussels,
fresh or frozen) |
5 ´ 105/g |
| West Virginia |
- |
- |
| Wisconsin |
- |
- |
| Wyoming |
- |
- |
(AFDO, 1998)
Contents
Recommended Microbiological Limits
Contents
ICMSF
Table 9-3. Recommended microbiological limits for fish and fishery products (ICMSF,
1986).
| Product |
n1 |
c2 |
Bacteria/g
or cm2 |
m3 |
M 4 |
| Fresh and frozen fish and cold-smoked fish |
5 |
3 |
5 ´ 105 |
107 |
| Precooked breaded fish |
5 |
2 |
5 ´ 105 |
107 |
| Frozen raw crustaceans |
5 |
3 |
106 |
107 |
| Frozen cooked crustaceans |
5 |
2 |
5 ´ 105 |
107 |
| Cooked, chilled, and frozen crabmeat |
5 |
2 |
105 |
106 |
| Fresh and frozen bivalve molluscs |
5 |
0 |
5 ´ 105 |
- |
1
Number of representative sample units.
2 Maximum number of acceptable sample units with bacterial counts between m and
M.
3 Maximum recommended bacterial counts for good quality products.
4 Maximum recommended bacterial counts for marginally acceptable quality
products.
Plate counts below "m" are considered good quality. Plate counts between
"m" and "M" are considered marginally acceptable quality, but can be
accepted if the number of samples does not exceed "c." Plate counts at or above
"M" are considered unacceptable quality (ICMSF, 1986).
Contents
Analytical Procedures
Contents
Food Sampling and Preparation of Sample Homogenate (Andrews and June,
1998)
The adequacy and condition of the sample or specimen received for examination are of
primary importance. If samples are improperly collected and mishandled or are not
representative of the sampled lot, the laboratory results will be meaningless. Because
interpretations about a large consignment of food are based on a relatively small sample
of the lot, established sampling procedures must be applied uniformly. A representative
sample is essential when pathogens or toxins are sparsely distributed within the food or
when disposal of a food shipment depends on the demonstrated bacterial content in relation
to a legal standard.
The number of units that comprise a representative sample from a designated lot of a
food product must be statistically significant. The composition and nature of each lot
affects the homogeneity and uniformity of the total sample mass. The proper statistical
sampling procedure, according to whether the food is solid, semisolid, viscous, or liquid,
must be determined by the collector at the time of sampling by using the Investigations
Operation Manual (FDA, 1993). Sampling and sample plans are discussed in detail in
ICMSF (1986).
Whenever possible, submit samples to the laboratory in the original unopened
containers. If products are in bulk or in containers too large for submission to the
laboratory, transfer representative portions to sterile containers under aseptic
conditions. There can be no compromise in the use of sterile sampling equipment and the
use of aseptic technique. Sterilize one-piece stainless steel spoons, forceps, spatulas,
and scissors in an autoclave or dry-heat oven. Use of a propane torch or dipping the
instrument in alcohol and igniting is dangerous and may be inadequate for sterilizing
equipment.
Use containers that are clean, dry, leak-proof, wide-mouthed, sterile, and of a size
suitable for samples of the product. Containers such as plastic jars or metal cans that
are leak-proof may be hermetically sealed. Whenever possible, avoid glass containers,
which may break and contaminate the food product. For dry materials, use sterile metal
boxes, cans, bags, or packets with suitable closures. Sterile plastic bags (for dry,
unfrozen materials only) or plastic bottles are useful containers for line samples. Take
care not to overfill bags or permit puncture by wire closure. Identify each sample unit
(defined later) with a properly marked strip of masking tape. Do not use a felt pen on
plastic because the ink might penetrate the container. Whenever possible, obtain at least
100 g for each sample unit. Submit open and closed controls of sterile containers with the
sample.
Deliver samples to the laboratory promptly with the original storage conditions
maintained as nearly as possible. When collecting liquid samples, take an additional
sample as a temperature control. Check the temperature of the control sample at the time
of collection and on receipt at the laboratory. Make a record for all samples of the times
and dates of collection and of arrival at the laboratory. Dry or canned foods that are not
perishable and are collected at ambient temperatures need not be refrigerated. Transport
frozen or refrigerated products in approved insulated containers of rigid construction so
that they will arrive at the laboratory unchanged. Collect frozen samples in pre-chilled
containers.
Place containers in a freezer long enough to chill them thoroughly. Keep frozen samples
solidly frozen at all times. Cool refrigerated samples, except shellfish and shell stock,
in ice at 0-4ºC and transport them in a sample chest with suitable refrigerant capable of
maintaining the sample at 0-4ºC until arrival at the laboratory. Do not freeze
refrigerated products. Unless otherwise specified, refrigerated samples should not be
analyzed more than 36 h after collection. Special conditions apply to the collection and
storage of shucked, unfrozen shellfish and shell stock (APHA, 1985). Pack samples of
shucked shellfish immediately in crushed ice (no temperature specified) until analyzed;
keep shell stock above freezing but below 10ºC. Examine refrigerated shellfish and shell
stock within 6 h of collection but in no case more than 24 h after collection. Further
details on sample handling and shipment may be found in the Investigations Operation
Manual (FDA, 1993) and the Laboratory Procedures Manual (FDA, 1989). The Investigations
Operation Manual (FDA, 1993) contains sampling plans for various microorganisms. Some
of those commonly used are presented here.
- Sampling plans
- Aerobic plate counts, total coliforms, fecal coliforms, Escherichia coli (including
enteropathogenic strains), Staphylococcus spp., Vibrio spp., Shigella spp.,
Campylobacter spp., Yersinia spp., Bacillus cereus, and Clostridium
perfringens
- Sample collection.
From any lot of food, collect ten 8 ounce (227 g) subsamples (or
retail packages) at random. Do not break or cut larger retail packages to obtain an 8
ounce (227 g) subsample. Collect the intact retail unit as the subsample even if it is
larger than 8 ounce (227 g).
- Sample analysis.
Analyze samples as indicated in current compliance programs.
- Equipment and materials
- Mechanical blender.
Several types are available. Use blender that has several
operating speeds or rheostat. The term "high-speed blender" designates mixer
with 4 canted, sharp-edge, stainless steel blades rotating at bottom of 4 lobe jar at
10,000-12,000 rpm or with equivalent shearing action. Suspended solids are reduced to fine
pulp by action of blades and by lobular container, which swirls suspended solids into
blades. Waring blender, or equivalent, meets these requirements.
- Sterile glass or metal high-speed blender jar.
1000 ml, with cover, resistant to
autoclaving for 60 min at 121ºC.
- Balance, with weights.
2000 g capacity, sensitivity of 0.1 g.
- Sterile beakers.
250 ml, low-form, covered with aluminum foil.
- Sterile graduated pipets.
1.0 and 10.0 ml.
- Butterfield's phosphate-buffered dilution water (R11)
.
Sterilized in bottles to yield final volume of 90 ± 1 ml.
- Sterile knives, forks, spatulas, forceps, scissors, tablespoons, and tongue depressors.
For
sample handling.
- Receipt of samples
- The official food sample is collected by the FDA inspector or investigator. As soon as
the sample arrives at the laboratory, the analyst should note its general physical
condition. If the sample cannot be analyzed immediately, it should be stored as described
later. Whether the sample is to be analyzed for regulatory purposes, for investigation of
a foodborne illness outbreak, or for a bacteriological survey, strict adherence to the
recommendations described here is essential.
- Condition of sampling container
. Check sampling containers for gross physical
defects. Carefully inspect plastic bags and bottles for tears, pinholes, and puncture
marks. If sample units were collected in plastic bottles, check bottles for fractures and
loose lids. If plastic bags were used for sampling, be certain that twist wires did not
puncture surrounding bags. Any cross-contamination resulting from one or more of above
defects would invalidate the sample, and the collecting district should be notified (see
3-e, below).
- Labeling and records
. Be certain that each sample is accompanied by a completed copy
of the Collection Report (Form FD-464) and officially sealed with tape (FD-415a) bearing
the sample number, collecting official's name, and date. Assign each sample unit an
individual unit number and analyze as a discrete unit unless the sample is composited as
described previously in this chapter.
- Adherence to sampling plan
. Most foods are collected under a specifically designed
sampling plan in one of several ongoing compliance programs. Foods to be examined for Salmonella,
however, are sampled according to a statistically based sampling plan designed exclusively
for use with this pathogen. Depending on the food and the type of analysis to be
performed, determine whether the food has been sampled according to the most appropriate
sampling plan.
- Storage
. If possible, examine samples immediately upon receipt. If analysis must be
postponed, however, store frozen samples at -20°C until examination. Refrigerate unfrozen
perishable samples at 0-4°C not longer than 36 h. Store nonperishable, canned, or
low-moisture foods at room temperature until analysis.
- Notification of collecting district
. If a sample fails to meet the above criteria
and is therefore not analyzed, notify the collecting district so that a valid sample can
be obtained and the possibility of a recurrence reduced.
- Thawing
Use aseptic technique when handling product. Before handling or analysis of sample,
clean immediate and surrounding work areas. In addition, swab immediate work area with
commercial germicidal agent. Preferably, do not thaw frozen samples before analysis. If
necessary to temper a frozen sample to obtain an analytical portion, thaw it in the
original container or in the container in which it was received in the laboratory.
Whenever possible, avoid transferring the sample to a second container for thawing.
Normally, a sample can be thawed at 2-5ºC within 18 h. If rapid thawing is desired, thaw
the sample at less than 45ºC for not more than 15 min. When thawing a sample at elevated
temperatures, agitate the sample continuously in thermostatically controlled water bath.
- Mixing
Various degrees of non-uniform distribution of microorganisms are to be expected in
any food sample. To ensure more even distribution, shake liquid samples thoroughly and, if
practical, mix dried samples with sterile spoons or other utensils before withdrawing the
analytical unit from a sample of 100 g or greater. Use a 50 g analytical unit of liquid or
dry food to determine aerobic plate count value and most probable number of coliforms.
Other analytical unit sizes (e.g., 25 g for Salmonella) may be recommended,
depending on specific analysis to be performed. Use analytical unit size and diluent
volume recommended for appropriate Bacteriological Analytical Manual method being
used. If contents of package are obviously not homogeneous (e.g., a frozen dinner),
macerate entire contents of package and withdraw the analytical unit, or, preferably,
analyze each different food portion separately, depending on purpose of test.
- Weighing
Tare high-speed blender jar; then aseptically and accurately (± 0.1 g) weigh
unthawed food (if frozen) into jar. If entire sample weighs less than the required amount,
weigh portion equivalent to one-half of sample and adjust amount of diluent or broth
accordingly. Total volume in blender must completely cover blades.
- Blending and diluting of samples requiring enumeration of microorganisms
- All foods other than nut meat halves and larger pieces, and nut meal
. Add 450 ml
Butterfield's phosphate-buffered dilution water to blender jar containing 50 g analytical
unit and blend 2 min. This results in a dilution of 10-1. Make dilutions of
original homogenate promptly, using pipets that deliver required volume accurately. Do not
deliver less than 10% of total volume of pipet. For example, do not use pipet with
capacity greater than 10 ml to deliver 1 ml volumes; for delivering 0.1 ml volumes, do not
use pipet with capacity greater than 1.0 ml. Prepare all decimal dilutions with 90 ml of
sterile diluent plus 10 ml of previous dilution, unless otherwise specified. Shake all
dilutions vigorously 25 times in 30 cm (1 foot) arc in 7 s. Not more than 15 min should
elapse from the time sample is blended until all dilutions are in appropriate media.
- Nut meat halves and larger pieces
. Aseptically weigh 50 g analytical unit into
sterile screw-cap jar. Add 50 ml diluent (7-a, above) and shake vigorously 50 times
through 30 cm arc to obtain 100 dilution. Let stand 3-5 min and shake 5 times
through 30 cm arc to resuspend just before making serial dilutions and inoculations.
- Nut meal
. Aseptically weigh 10 g analytical unit into sterile screw-cap jar. Add 90
ml of diluent (7-a, above) and shake vigorously 50 times through 30 cm arc to obtain 10-1
dilution. Let stand 3-5 min and shake 5 times through 30 cm arc to resuspend just before
making serial dilutions and inoculations.
Contents
Aerobic plate count (Maturin and Peeler, 1998)
The aerobic plate count (APC) is intended to indicate the level of microorganisms in a
product. Detailed procedures for determining the APC of foods have been developed by the
Association of Official Analytical Chemists (AOAC, 1990) and the American Public Health
Association (APHA, 1984). The conventional plate count method for examining frozen,
chilled, precooked, or prepared foods, outlined below, conforms to AOAC Official
Methods of Analysis, sec. 966.23, with one procedural change (966.23C).
The suitable colony counting range is 25-250 (Tomasiewicz et al., 1980). The automated
spiral plate count method for the examination of foods and cosmetics, outlined below,
conforms to AOAC Official Methods of Analysis, sec. 977.27 (Gilchrist et
al., 1977). For procedural details of the standard plate count, see APHA (1993).
Guidelines for calculating and reporting plate counts have been changed to conform with
the anticipated changes in the 16th edition of Standard Methods for the Examination of
Dairy Products (APHA, 1993) and the International Dairy Federation (IDF)
procedures (IDF, 1987).
Contents
Conventional Plate Count Method
- Equipment and materials
- Work area, level table with ample surface in room that is clean, well-lighted (100
foot-candles at working surface) and well-ventilated, and reasonably free of dust and
drafts. The microbial density of air in working area, measured in fallout pour plates
taken during plating, should not exceed 15 colonies/plate during 15 min exposure.
- Storage space, free of dust and insects and adequate for protection of equipment and
supplies
- Petri dishes, glass or plastic (at least 15 ´ 90 mm)
- Pipets with pipet aids (no mouth pipetting) or pipettors, 1, 5, and 10 ml, graduated in
0.1 ml units
- Dilution bottles, 6 ounces (160 ml), borosilicate-resistant glass, with rubber stoppers
or plastic screw caps
- Pipet and petri dish containers, adequate for protection
- Circulating water bath, for tempering agar, thermostatically controlled to 45 ± 1ºC
- Incubator, 35 ± 1ºC; milk, 32 ± 1ºC
- Colony counter, dark-field, Quebec, or equivalent, with suitable light source and grid
plate
- Tally register
- Dilution blanks, 90 ± 1 ml Butterfield's phosphate-buffered dilution water (R11); milk, 99 ± 2 ml
- Plate count agar (standard methods) (M124)
- Refrigerator, to cool and maintain samples at 0-5ºC; milk, 0-4.4ºC
- Freezer, to maintain frozen samples from -15 to –20ºC
- Thermometers (mercury) appropriate range; accuracy checked with a thermometer certified
by the National Institute of Standards and Technology (NIST)
Procedure for analysis of frozen, chilled, precooked, or prepared foods
Using separate sterile pipets, prepare decimal dilutions of 10-2,
10-3, 10-4, and others as appropriate, of food homogenate (see
"Food Sampling and Preparation of Sample Homogenate" for sample preparation) by
transferring 10 ml of previous dilution to 90 ml of diluent. Avoid sampling foam. Shake
all dilutions 25 times in 30 cm (1 foot) arc within 7 s. Pipet 1 ml of each dilution into
separate, duplicate, appropriately marked petri dishes. Reshake dilution bottle 25 times
in 30 cm arc within 7 s if it stands more than 3 min before it is pipetted into petri
dish. Add 12-15 ml plate count agar (cooled to 45 ± 1ºC) to each plate within 15 min of
original dilution. For milk samples, pour an agar control, pour a dilution water control
and pipet water for a pipet control. Add agar to the latter two for each series of
samples. Add agar immediately to petri dishes when sample diluent contains hygroscopic
materials, e.g., flour and starch. Pour agar and dilution water control plates for each
series of samples. Immediately mix sample dilutions and agar medium thoroughly and
uniformly by alternate rotation and back-and-forth motion of plates on flat level surface.
Let agar solidify. Invert solidified petri dishes, and incubate promptly for 48 ± 2 h at
35ºC. Do not stack plates when pouring agar or when agar is solidifying.
Guidelines for calculating and reporting APCs in uncommon cases
Official Methods of Analysis (AOAC, 1990) does not provide guidelines for
counting and reporting plate counts, whereas Standard Methods for the Examination of
Dairy Products, 16th ed. (APHA, 1993) presents detailed guidelines; for uniformity,
therefore, use APHA guidelines as modified (IDF, 1987; Niemela, 1983). Report all aerobic
plate counts (APHA, 1993) computed from duplicate plates. For milk samples, report all
aerobic plate (APHA, 1992) counts computed from duplicate plates containing less than 25
colonies as less than 25 estimated count. Report all aerobic plate counts (APHA, 1993)
computed from duplicate plates containing more than 250 colonies as estimated counts.
Counts outside the normal 25-250 range may give erroneous indications of the actual
bacterial composition of the sample. Dilution factors may exaggerate low counts (less than
25), and crowded plates (greater than 250) may be difficult to count or may inhibit the
growth of some bacteria, resulting in a low count. Report counts less than 25 or more than
250 colonies as estimated aerobic plate counts (EAPC). Use the following guide:
- Normal plates (25-250)
. Select spreader-free plate(s). Count all colony forming
units (CFU), including those of pinpoint size, on selected plate(s). Record dilution(s)
used and total number of colonies counted.
- Plates with more than 250 colonies
. When number of CFU per plate exceeds 250, for
all dilutions, record the counts as too numerous to count (TNTC) for all but the plate
closest to 250, and count CFU in those portions of plate that are representative of colony
distribution. See APHA (1993) for detailed guidelines. Mark calculated APC with EAPC to
denote that it was estimated from counts outside 25-250 per plate range (see 4-3).
- Spreaders
. Spreading colonies are usually of 3 distinct types: 1) a chain of
colonies, not too distinctly separated, that appears to be caused by disintegration of a
bacterial clump; 2) one that develops in film of water between agar and bottom of dish;
and 3) one that forms in film of water at edge or on surface of agar. If plates prepared
from sample have excessive spreader growth so that (a) area covered by spreaders,
including total area of repressed growth, exceeds 50% of plate area, or (b) area of
repressed growth exceeds 25% of plate area, report plates as spreaders. When it is
necessary to count plates containing spreaders not eliminated by (a) or (b) above, count
each of the 3 distinct spreader types as one source. For the first type, if only one chain
exists, count it as a single colony. If one or more chains appear to originate from
separate sources, count each source as one colony. Do not count each individual growth in
such chains as a separate colony. Types 2 and 3 usually result in distinct colonies and
are counted as such. Combine the spreader count and the colony count to compute the APC.
- Plates with no CFU
. When plates from all dilutions have no colonies, report APC as
less than 1 times the corresponding lowest dilution used. Mark calculated APC with
asterisk to denote that it was estimated from counts outside the 25-250 per plate range.
When plate(s) from a sample are known to be contaminated or otherwise unsatisfactory,
record the result(s) as laboratory accident (LA).
Computing and recording counts (see IDF, 1987; Niemela, 1983)
To avoid creating a fictitious impression of precision and accuracy when computing APC,
report only the first two significant digits. Round off to two significant figures only at
the time of conversion to SPC. For milk samples, when plates for all dilutions have no
colonies, report APC as less than 25 colonies estimated count. Round by raising the second
digit to the next highest number when the third digit is 6, 7, 8, or 9 and use zeros for
each successive digit toward the right from the second digit. Round down when the third
digit is 1, 2, 3, or 4. When the third digit is 5, round up when the second digit is odd
and round down when the second digit is even.
Examples
Calculated Count |
APC |
12,700 |
13,000 |
12,400 |
12,000 |
15,500 |
16,000 |
14,500 |
14,000 |
- Plates with 25-250 CFU
- Calculate the APC as follows:
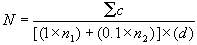
where
N = Number of colonies per ml or g of product
å c = Sum of all colonies on all plates counted
n1 = Number of plates in first dilution counted
n2 = Number of plates in second dilution counted
d = Dilution from which the first counts were obtained
Example
1:100 |
1:1,000 |
232, 244 |
33,28 |

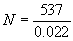
N = 24,409 = 24,000
- When counts of duplicate plates fall within and without the 25-250 colony range, use
only those counts that fall within this range.
All plates with fewer than 25 CFU. When plates from both dilutions yield fewer than
25 CFU each, record actual plate count but record the count as less than 25 x 1/d when d
is the dilution factor for the dilution from which the first counts were obtained.
Example
Colonies |
|
1:100 |
1:1,000 |
EAPC/ml (g) |
18 |
2 |
<2,500 |
0 |
0 |
<2,500 |
EAPC, estimated aerobic plate count.
All plates with more than 250 CFU. When plates from both 2 dilutions yield more than
250 CFU each (but fewer than 100/cm2), estimate the aerobic counts from the
plates (EAPC) nearest 250 and multiply by the dilution.
Example
Colonies |
|
1:100 |
1:1,000 |
EAPC/ml (g) |
TNTC |
640 |
640,000 |
TNTC, too numerous to count.
EAPC, estimated aerobic plate count.
All plates with spreaders and/or laboratory accident. Report respectively as
Spreader (SPR), or Laboratory Accident (LA).
All plates with more than an average of 100 CFU/cm2. Estimate the APC as
greater than 100 times the highest dilution plated, times the area of the plate. The
examples below have an average count of 110/cm2.
Example
Colonies/Dilution |
|
1:100 |
1:1,000 |
EAPCa/ml (g) |
TNTC |
7,150b |
>6,500,000 |
TNTC |
6,490c |
>5,900,000 |
a
Estimated aerobic plate count.
bBased on plate area of 65 cm2
cBased on plate area of 59 cm2
Contents
Spiral Plate Method
The spiral plate count (SPLC) method for microorganisms in milk, foods, and cosmetics
is an official method of the APHA (1993) and the AOAC (1990). In this method, a mechanical
plater inoculates a rotating agar plate with liquid sample. The sample volume dispensed
decreases as the dispensing stylus moves from the center to the edge of the rotating
plate. The microbial concentration is determined by counting the colonies on a part of the
petri dish where they are easily countable and dividing this count by the appropriate
volume. One inoculation determines microbial densities between 500 and 500,000
microorganisms/ml. Additional dilutions may be made for suspected high microbial
concentrations.
- Equipment and materials
- Spiral plater (Spiral Systems Instruments, Inc., 7830 Old Georgetown Road, Bethesda, MD
20814)
- Spiral colony counter (Spiral Systems) with special grid for relating deposited sample
volumes to specific portions of petri dishes
- Vacuum trap for disposal of liquids (2-4 liter vacuum bottle to act as vacuum reservoir
and vacuum source of 50-60 cm Hg)
- Disposable micro beakers, 5 ml
- Petri dishes, plastic or glass, 150 x 15 mm or 100 x 15 mm
- Plate count agar (standard methods) (M124)
- Calculator (optional), inexpensive electronic hand calculator is recommended
- Polyethylene bags for storing prepared plates
- Commercial sodium hypochlorite solution, about 5% NaOCl (bleach)
- Sterile dilution water
- Syringe, with Luer tip for obstructions in stylus; capacity not critical
- Work area, storage space, refrigerator, thermometers, tally, and incubator, as described
for Conventional Plate Count Method, above.
- Sodium hypochlorite solution (5.25%). Available commercially.
- Preparation of agar plates
Automatic dispenser with sterile delivery system is recommended to prepare agar
plates. Agar volume dispensed into plates is reproducible and contamination rate is low
compared to hand pouring of agar in open laboratory. When possible, use laminar airflow
hood along with automated dispenser. Pour same quantity of agar into all plates so that
same height of agar will be presented to spiral plater stylus tip to maintain contact
angle. Agar plates should be level during cooling.
The following method is suggested for prepouring agar plates: Use automatic dispenser
or pour constant amount (about 15 ml/100 mm plate; 50 ml/150 mm plate) of sterile agar at
60-70ºC into each petri dish. Let agar solidify on level surface with poured plates
stacked no higher than 10 dishes. Place solidified agar plates in polyethylene bags, close
with ties or heat-sealer, and store inverted at 0-4.4ºC. Bring prepoured plates to room
temperature before inoculation.
- Preparation of samples
As described in "Food Sampling and Preparation of Sample Homogenate,"
select that part of sample with smallest amount of connective tissues or fat globules.
- Description of spiral plater
Spiral plater inoculates surface of prepared agar plate to permit
enumeration of microorganisms in solutions containing between 500 and 500,000
microorganisms per ml. Operator with minimum training can inoculate 50 plates per h.
Within range stated, dilution bottles or pipets and other auxiliary equipment are not
required. Required bench space is minimal, and time to check instrument alignment is less
than 2 min. Plater deposits decreasing amount of sample in Archimedean spiral on surface
of prepoured agar plate. Volume of sample on any portion of plate is known. After
incubation, colonies appear along line of spiral. If colonies on a portion of plate are
sufficiently spaced from each other, count them on special grid which associates a
calibrated volume with each area. Estimate number of microorganisms in sample by dividing
number of colonies in a defined area by volume contained in same area. Studies have shown
the method to be proficient not only with milk (Donnelly et al., 1976) but also with other
foods (Jarvis et al., 1977; Zipkes et al., 1981.
- Plating procedure
Check stylus tip angle daily and adjust if necessary. (Use vacuum to hold
microscope cover slip against face of stylus tip; if cover slip plane is parallel at about
l mm from surface of platform, tip is properly oriented.) Liquids are moved through system
by vacuum. Clean stylus tip by rinsing for 1 second with sodium hypochlorite solution
followed by sterile dilution water for 1 second before sample introduction. This rinse
procedure between processing of each sample minimizes cross-contamination. After rinsing,
draw sample into tip of Teflon tubing by vacuum applied to 2-way valve. When tubing and
syringe are filled with sample, close valve attached to syringe. Place agar plate on
platform, place stylus tip on agar surface, and start motor. During inoculation, label
petri plate lid. After agar has been inoculated, stylus lifts from agar surface and spiral
plater automatically stops. Remove inoculated plate from platform and cover it. Move
stylus back to starting position. Vacuum-rinse system with hypochlorite and water, and
then introduce new sample. Invert plates and promptly place them in incubator for 48 ± 3
h at 35 ± 1ºC.
- Sterility controls
Check sterility of spiral plater for each series of samples by plating sterile
dilution water. CAUTION: Prepoured plates should not be contaminated by a surface
colony or be below room temperature (water can well up from agar). They should not be
excessively dry, as indicated by large wrinkles or glazed appearance. They should not have
water droplets on surface of agar or differences greater than 2 mm in agar depth, and they
should not be stored at 0-4.4ºC for longer than l month. Reduced flow rate through tubing
indicates obstructions or material in system. To clear obstructions, remove valve from
syringe, insert hand-held syringe with Luer fitting containing water, and apply pressure.
Use alcohol rinse to remove residual material adhering to walls of system. Dissolve
accumulated residue with chromic acid. Rinse well after cleaning.
- Counting grid
- Description
. Use same counting grid for both 100 and 150 mm petri dishes. A mask is
supplied for use with 100 mm dishes. Counting grid is divided into 8 equal wedges; each
wedge is divided by 4 arcs labeled l, 2, 3, and 4 from outside grid edge. Other lines
within these arcs are added for ease of counting. A segment is the area between 2 arc
lines within a wedge. Number of areas counted (e.g., 3) means number of segments counted
within a wedge. Spiral plater deposits sample on agar plate in the same way each time. The
grid relates colonies on spiral plate to the volume in which they were contained. When
colonies are counted with grid, sample volume becomes greater as counting starts at
outside edge of plate and proceeds toward center of plate.
- Calibration
. The volume of sample represented by various parts of the counting grid
is shown in operator's manual that accompanies spiral plater. Grid area constants have
been checked by the manufacturer and are accurate. To verify these values, prepare 11
bacterial concentrations in range of 106-103cells/ml by making 1:1
dilutions of bacterial suspension (use a nonspreader). Plate all Incubate both sets of
plates for 48 ± 3 h at 35 ± 1ºC. Calculate concentrations for each dilution. Count
spiral plates over grid surface, using counting rule of 20 (described in H, below),
and record number of colonies counted and grid area over which they were counted. Each
spiral colony count for a particular grid area, divided by aerobic count/ml for
corresponding spirally plated bacterial concentrations, indicates volume deposited on that
particular grid area. Use the following formula:
Volume (ml) for grid area = Spiral colonies counted in area/Bacterial count/ml (APC)
Example:
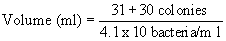
Volume (ml) = 0.0015 ml
To check total volume dispensed by spiral plater, weigh amount dispensed from stylus
tip. Collect in tared 5 ml plastic beaker and weigh on analytical balance (± 0.2 mg).
Examination and reporting of spiral plate counts
Counting rule of 20. After incubation, center spiral plate over grid by adjusting
holding arms on viewer. Choose any wedge and begin counting colonies from outer edge of
first segment toward center until 20 colonies have been counted. Complete by counting
remaining colonies in segment where 20th colony occurs. In this counting procedure,
numbers such as 3b, 4c (see Figure 9-1)
refer to area segments from outer edge of wedge to designated arc line. Any count
irregularities in sample composition are controlled by counting the same segments in the
opposite wedge and recording results. Example of spirally inoculated plate (see Figure 9-1) demonstrates method for determining
microbial count. Two segments of each wedge were counted on opposite sides of plate with
31 and 30 colonies, respectively. The sample volume contained in the darkened segments is
0.0015 ml. To estimate number of microorganisms, divide count by volume contained in all
segments counted. See example under Figure 9-1.
If 20 CFU are not within the 4 segments of the wedge, count CFU on entire plate. If the
number of colonies exceeds 75 in second, third, or fourth segment, which also contains the
20th colony, the estimated number of microorganisms will generally be low because of
coincidence error associated with crowding of colonies. In this case, count each
circumferentially adjacent segment in all 8 wedges, counting at least 50 colonies, e.g.,
if the first 2 segments of a wedge contain 19 colonies and the third segment contains the
20th and 76th (or more), count colonies in all circumferentially adjacent first and second
segments in all 8 wedges. Calculate contained volume in counted segments of wedges and
divide into number of colonies.
When fewer than 20 colonies are counted on the total plate, report results as
"less than 500 estimated SPLC per ml." If colony count exceeds 75 in first
segment of wedge, report results as "greater than 500,000 estimated SPLC per
ml." Do not count spiral plates with irregular distribution of colonies caused by
dispensing errors. Report results of such plates as laboratory accident (LA). If spreader
covers entire plate, discard plate. If spreader covers half of plate area, count only
those colonies that are well distributed in spreader-free areas.
Compute SPLC unless restricted by detection of inhibitory substances in sample,
excessive spreader growth, or laboratory accidents. Round off counts as described in 4,
above. Report counts as SPLC or estimated SPLC per ml.
Contents
Other APC methods
- Aerobic plate count in foods: Dry rehydratable film (AOAC, 1995a).
- Aerobic plate count in foods: Hydrophobic grid filter method (AOAC, 1995b).
- Aerobic plate count: Pectin gel method (AOAC, 1995c).
Contents
Commercial Test Products
Table 9-4. Commercial APC test products.
Test |
Analytical Technique |
Approx. Total Test
Time1 |
Supplier |
3M PetrifilmTM
Aerobic Count Plate2 |
Dry rehydratable film method |
48 h |
3M Microbiology Products
3M Center, Building 275-5W-05
St. Paul, MN 55144-1000
Phone: 800/228-3957
E-mail: microbiology@mmm.com |
| bio-spoTM Solar Cult® Flex
Paddles |
Gellified agar slide |
24 h |
Applied Research Institute
Contact: Trevor Hopkins
3N Simm Ln.
Newton, CT 06470
Phone: 888/324-7900
E-mail: sales@arillc.com
Web: www.arillc.com |
| BioProbe® ATP Detection System |
ATP bioluminescence |
1 min |
Contamination Sciences LLC
Contact: Robert Steinhauser
4230 East Towne Blvd., Suite 191
Madison, WI 53704
Phone: 608/825-6125
E-mail: info@contam-sci.com
Web: www.contam-sci.com |
| HYGI-PRO |
Chemical Analysis visual color change |
10 min |
Contamination Sciences LLC
Contact: Robert Steinhauser
4230 East Towne Blvd., Suite 191
Madison, WI 53704
Phone: 608/825-6125
E-mail: info@contam-sci.com
Web: www.contam-sci.com |
| Hygicult®TPC (Total Plate Count) |
Gellified agar
slide |
24 h |
Neogen Corporation
620 Lesher Pl.
Lansing, MI 48912
Phone: 517/372-9200
E-mail: NeogenCorp@aol.com
Web: www.neogen.com |
| ISO-GRID Method for Aerobic Plate Count using
TSAF Agar2 |
Membrane filtration using culture medium with
non-inhibitory dye to enhance colony appearance |
48 h |
QA Life Sciences, Inc.
6645 Nancy Ridge Dr.
San Diego, CA 92121
Phone: 800/788-4446; 858/622-0560
E-mail: bugsy@qalife.com |
| SimPlateTM for Total
Plate Count2 |
84/198 MPN well plates/UV fluorescence
|
24 h |
IDEXX Laboratories, Inc.
Contact: Greg Getchell
One Idexx Dr.
Westbrook, ME 04092
Phone: 800-321-0207; 207/856-0580
E-mail: greg-getchell@idexx.com
Web: www.idexx.com/fed/home/start.asp |
| Total Microbe Hunter |
Selective media with color indicator that
changes based on approximate TPC |
30 min for 108 organisms
10 h for 101 organisms |
Contamination Sciences LLC
Contact: Robert Steinhauser
4230 East Towne Blvd., Suite 191
Madison, WI 53704
Phone: 608/825-6125
E-mail: bsteinha@contam-sci.com |
1Includes enrichment
2AOAC Approved
Table 9-5. Sanitation test kits.
Test |
Used to Identify |
Analytical
Technique |
Approx. Total Test
Time |
Supplier |
| AssureSwab visual swab test |
Protein residue |
Visual chemical assay |
10 min |
Biocontrol Systems, Inc.
Contact: Robin Forgey
12822 SE 32nd St.
Bellevue, WA 98005
Phone: 425/603-1123
E-mail: info@rapidmethods.com
Web: rapidmethods.com |
| BIOPROBE® |
Bacteria/food residue |
ATP Bioluminescence |
1 min |
Contamination Sciences LLC
Contact: Robert Steinhauser
4230 East Towne Blvd., Ste. 191
Madison, WI 53704
Phone: 608/825-6125
E-mail: bsteinha@contam-sci.com |
| Hy-Lite®ATP Hygiene Monitoring
System |
Bacteria/food residue |
ATP Bioluminescence |
2 min |
Neogen Corporation
620 Lesher Pl.
Lansing, MI 48912
Phone: 517/372-9200
E-mail: NeogenCorp@aol.com
Web: www.neogen.com |
| LighteningTM |
Bacteria/food residue |
ATP Bioluminescence |
11 s |
IDEXX Laboratories, Inc.
Contact: Greg Getchell
One Idexx Dr.
Westbrook, ME 04092
Phone: 207/856-0580
E-mail: greg-getchell@idexx.com
Web: www.idexx.com/fed/home/start.asp |
| PocketSwab with Charm LUM-T Meter |
Bacteria/food residue |
ATP Bioluminescence |
30 s |
Charm Sciences, Inc.
36 Franklin St.
Malden, MA 02148-4120
Phone: 781/322-1523
E-mail: charm1@charm.com
Web: www.charm.com |
| systemSure |
Bacteria/food residue |
ATP Bioluminescence |
2 min |
Becton Dickinson Microbiology Systems
7 Loveton Circle
Sparks, MD 21152
Phone: 410/316-4472 |
Contents
References
AFDO. 1998. State and federal microbiological standards and guidelines. Association of
Food and Drug Officials, York, PA.
Andrews, W.H., and June, G.A. 1998. Food sampling and preparation of sample homogenate,
Ch. 1. In Food and Drug Administration Bacteriological Analytical Manual, 8th ed.
(revision A), (CD-ROM version). R.L. Merker (Ed.). AOAC International, Gaithersburg, MD.
Andrews, W.H., Hammack, T.S., and Amaguana, R.M. 1998. Salmonella. Ch. 5. In Food
and Drug Administration Bacteriological Analytical Manual, 8th ed. (revision A),
(CD-ROM version). R.L Merker (Ed.). AOAC International, Gaithersburg, MD.
AOAC. 1990. Official Methods of Analysis of AOAC International, 15th ed. AOAC
International, Gaithersburg, MD.
AOAC. 1995a. Aerobic plate count in foods: Dry rehydratable film. Sec. 17.02.07, Method
990.12. In Official Methods of Analysis of AOAC International, 16th ed., P.A.
Cunniff (Ed.), 10-11. AOAC International, Gaithersburg, MD.
AOAC. 1995b. Aerobic plate count in foods: Hydrophobic grid filter method. Sec.
17.2.05, Method 986.32. In Official Methods of Analysis of AOAC International,
16th ed., P.A. Cunniff (Ed.), 8-10. AOAC International, Gaithersburg, MD.
AOAC. 1995c. Aerobic plate count: Pectin gel method. Sec. 17.02.06, Method 988.18. In Official
Methods of Analysis of AOAC International, 16th ed., P.A. Cunniff (Ed.), 10. AOAC
International, Gaithersburg, MD.
AOAC. 1995d. Official Methods of Analysis of AOAC International, 16th ed., P.A.
Cunniff (Ed.). AOAC International, Gaithersburg, MD.
APHA. 1984. Compendium of Methods for the Microbiological Examination of Foods,
2nd ed. American Public Health Association, Washington, DC.
APHA. 1985. Laboratory Procedures for the Examination of Seawater and Shellfish,
5th ed. American Public Health Association, Washington, DC.
APHA. 1993. Standard Methods for the Examination of Dairy Products, 16th ed.
American Public Health Association, Washington, DC.
Donnelly, C.B., J.E. Gilchrist, J.T. Peeler, and J.E. Campbell. 1976. Spiral plate
count method for the examination of raw and pasteurized milk. Appl. Environ. Microbiol.
32:21-27.
FDA. 1978. Product code: Human foods. In EDRO Data Codes Manual. Food and Drug
Administration, Rockville, MD.
FDA. 1989. Laboratory Procedures Manual. Food and Drug Administration,
Rockville, MD.
FDA. 1993. Investigations Operations Manual. Food and Drug Administration,
Rockville, MD.
FDA. 1996a. Raw breaded shrimp – Microbiological criteria for evaluating
compliance with current good manufacturing practice regulations (CPG 7108.25). Section
540.420. Compliance Policy Guides, (August 1996 ed.), updated through October 31,
1996. Department of Health and Human Services, Public Health Service, Food and Drug
Administration.
FDA. 1998. FDA & EPA guidance levels. Appendix 5. In Fish and Fishery Products
Hazards and Controls Guide, 2nd ed., p. 245-248. Department of Health and
Human Services, Public Health Service, Food and Drug Administration, Center for Food
Safety and Applied Nutrition, Office of Seafood, Washington, DC.
Gilchrist, J.E., Donnelly, C.B., Peeler, J.T., and Campbell, J.E. 1977. Collaborative
study comparing the spiral plate and aerobic plate count methods. J. Assoc. Off. Anal.
Chem. 60:807-812.
ICMSF. 1986. Microorganisms in Foods. 2. Sampling For Microbiological Analysis:
Principles and Specific Applications, 2nd ed. University of Toronto Press, Buffalo,
NY.
IDF. 1987. Milk and milk products: Enumeration of microorganisms—Colony count at
3ºC. Provisional IDF Standard 100A. International Dairy Federation, Brussels, Belgium.
Jarvis, B., V.H. Lach, and J.M. Wood. 1977. Evaluation of the spiral plate maker for
the enumeration of microorganisms in foods. J. Appl. Bacteriol. 43:149-157.
Maturin, L.J. and Peeler, J.T. 1998. Aerobic plate count. Ch. 3. In Food and Drug
Administration Bacteriological Analytical Manual, 8th ed. (revision A), (CD-ROM
version). R.L. Merker (Ed.). AOAC International, Gaithersburg, MD.
NAS. 1969. An evaluation of the Salmonella problem. National Academy of
Sciences, Washington, DC.
Nickelson, R. and Finne, G. 1992. Fish, crustaceans, and precooked seafoods. Ch. 47. In
Compendium of Methods for the Microbiological Examination of Foods, 3rd
ed., C. Vanderzant and D. F. Splittstoesser (Ed.), p. 875-895. American Public Health
Association, Washington, DC.
Niemela, S. 1983. Statistical evaluation of results from the quantitative
microbiological examinations. Report No. 1, 2nd ed. Nordic Committee on Food
Analysis, Uppsala, Sweden.
Tomasiewicz, M.R., Hotchkiss, D.K., Reinbold, G.W., Read, R.B. Jr., and Hartman, P.A.
1980. The most suitable number of colonies on plates for counting. J. Food Protect.
43:282-286.
Zipkes, M.R., J.E. Gilchrist, and J.T. Peeler. 1981. Comparison of yeast and mold
counts by spiral, pour, and streak plate methods. J. Assoc. Off. Anal. Chem. 64:1465-1469.
![]()
![]()
![]()